
A simple representation of the sodium atom. Download Scientific Diagram
The chemical abbreviation for sodium was first published by Jöns Jakob Berzelius in his system of atomic symbols. It is a contraction of the element's new Latin name natrium, which refers to the Egyptian natron, a natural mineral salt primarily made of hydrated sodium carbonate. In 1807, Sir Humphry Davy isolated sodium for the first time by electrolysis of dried sodium hydroxide, which had.

The sodium atom YouTube
Periodic Table element Summary Sodium Sodium is a chemical element with symbol Na and atomic number 11. Classified as a n alkali metal, Sodium is a solid at room temperature. 11 Na Sodium View All Properties H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga
Atomic Structure Electrical Revolution
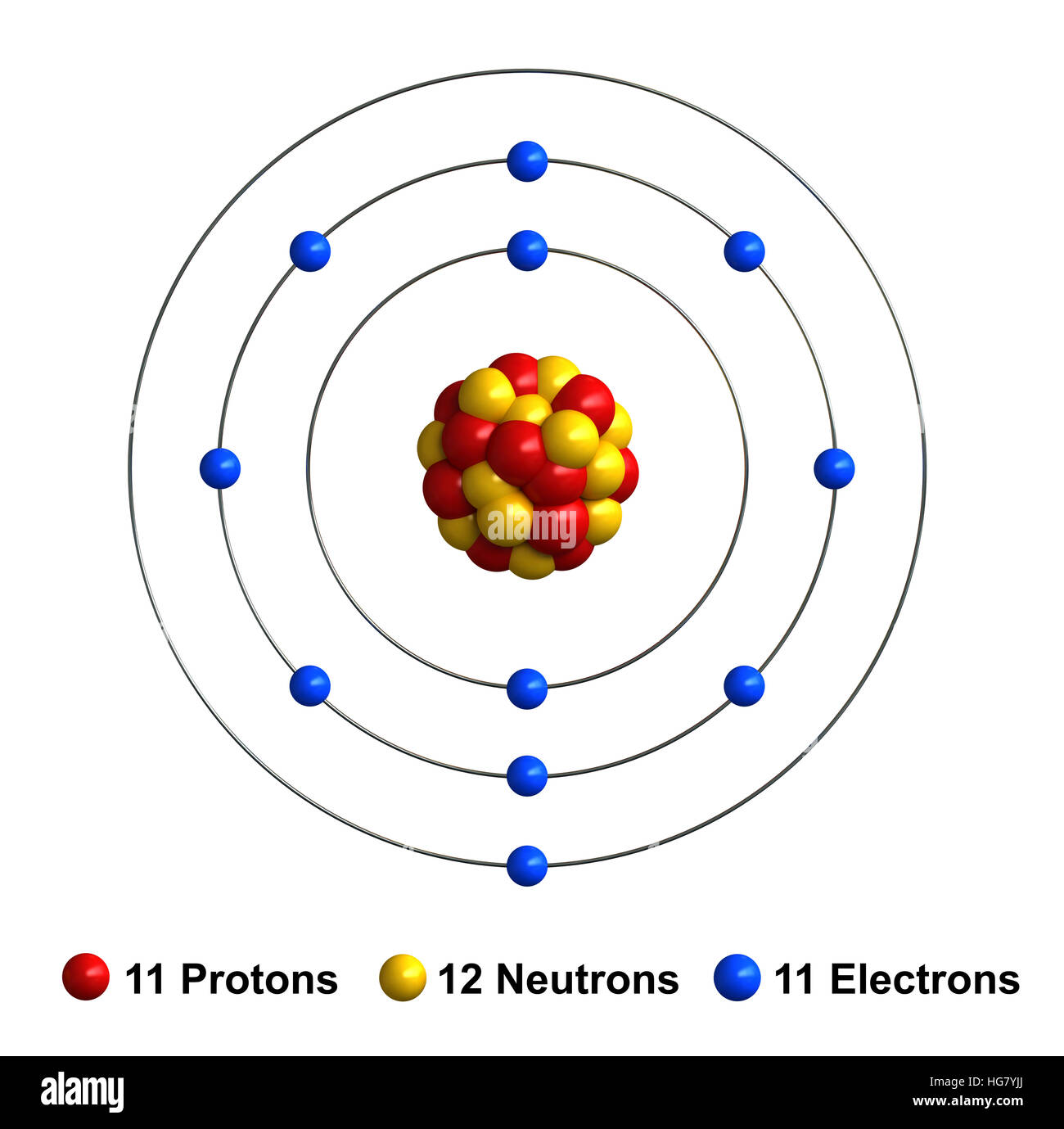
Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.

FileElectron shell 011 sodium.png Wikimedia Commons
Protons and Neutrons in Sodium. Sodium is a chemical element with atomic number 11 which means there are 11 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

Sodium Na (Element 11) of Periodic Table NewtonDesk
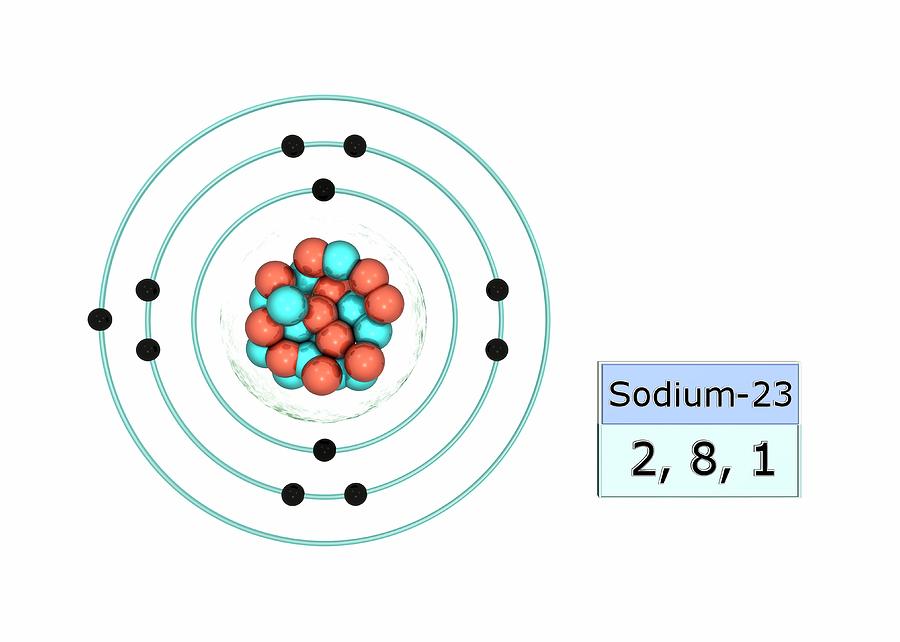
Atomic no. Element Shorthand Electron Configuration Full Electron Configuration Electron shell arrangement; 1: Electron configuration of Hydrogen (H) 1s 1: 1s 1: 1: 2: Electron configuration of Helium (He) 1s 2: 1s 2: 2: 3: Electron configuration of Lithium (Li) [He] 2s 1: 1s 2 2s 1: 2, 1: 4: Electron configuration of Beryllium (Be) [He] 2s 2.
Sodium Electron Configuration Electron Configuration Sodium What is the ground state
Chemistry of Sodium (Z=11) Sodium is metallic element found in the first group of the periodic table. As the sixth most abundant element in the Earth's crust, sodium compounds are commonly found dissolved in the oceans, in..
Sodium Electron Configuration Photograph by Photo Libary Pixels
Molecular Formula Na Synonyms 7440-23-5 Na Sodium Natrium Sodio View More. Molecular Weight 22.9897693 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Element Name Sodium Dates Create: 2004-09-16 Modify: 2023-12-23 Description Sodium appears as a silvery soft metal that becomes grayish white upon exposure to air.
:max_bytes(150000):strip_icc()/sodiumatom-58b602715f9b5860464c7a22.jpg)
Atom Diagrams Electron Configurations of the Elements
Name: Sodium Symbol: Na Atomic Number: 11 Atomic Mass: 22.98977 amu Melting Point: 97.72 °C (370.87 K, 207.9 °F) Boiling Point: 883 °C (1156 K, 1621 °F) Number of Protons/Electrons: 11 Number of Neutrons: 12 Classification: Alkali Metal Crystal Structure: Cubic Density @ 293 K: 0.971 g/cm 3 Color: silvery Atomic Structure
Sodium Electron Configuration Electron Configuration Sodium What is the ground state
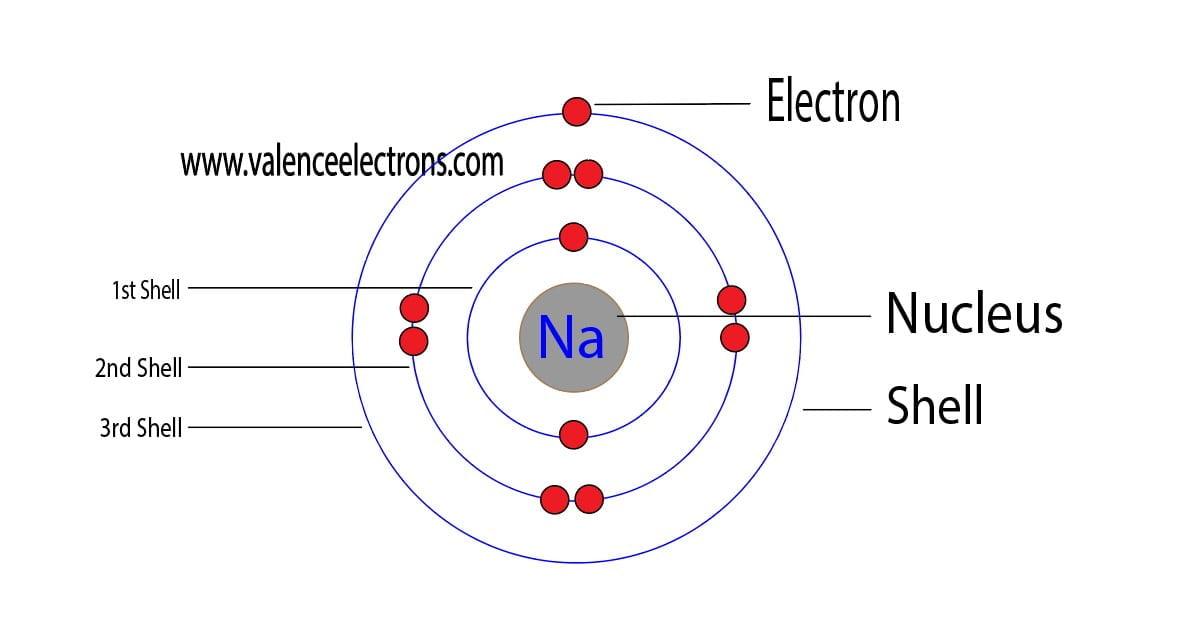
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.

Atom Sodium Model Stock Illustration Download Image Now iStock
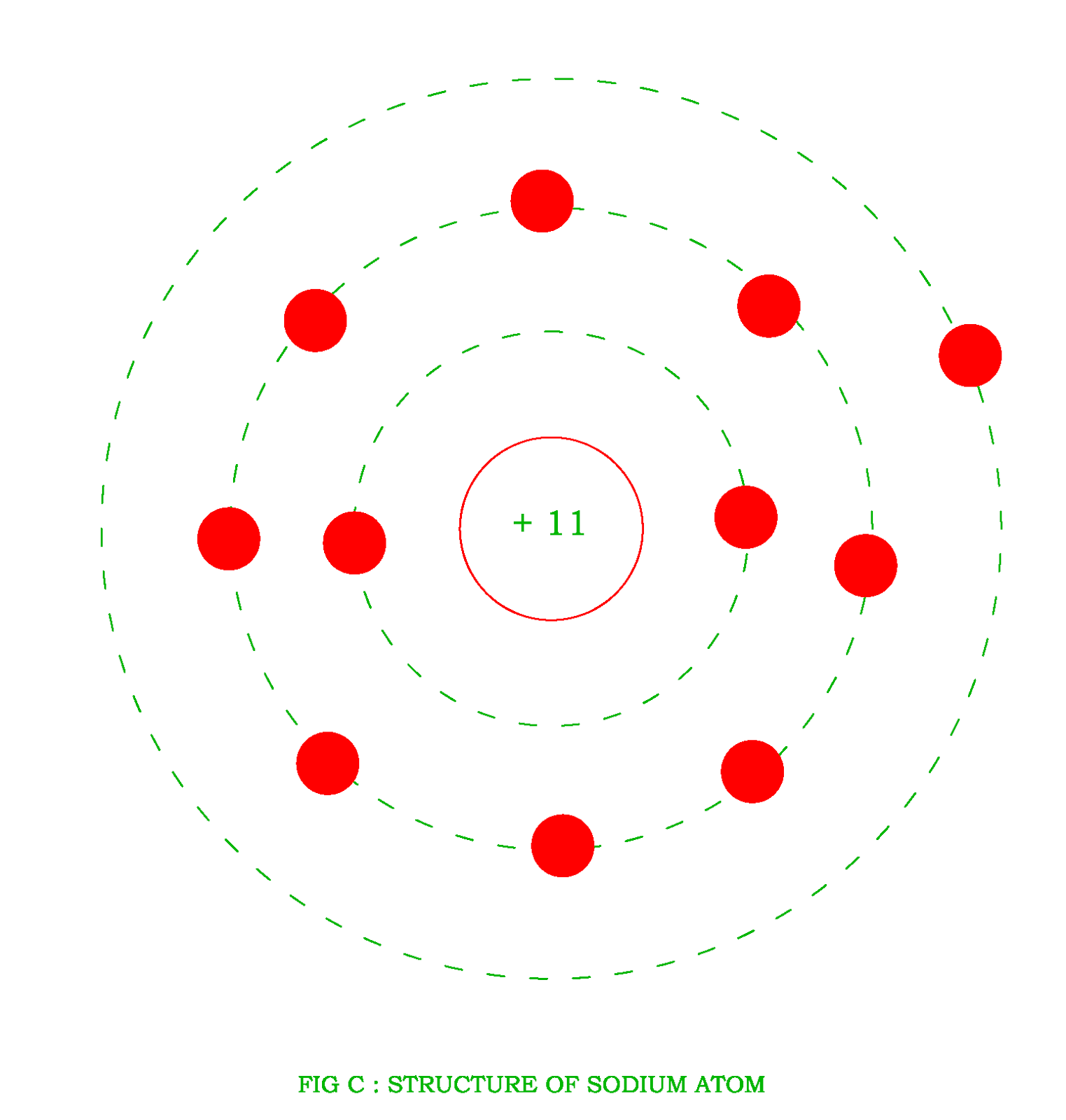
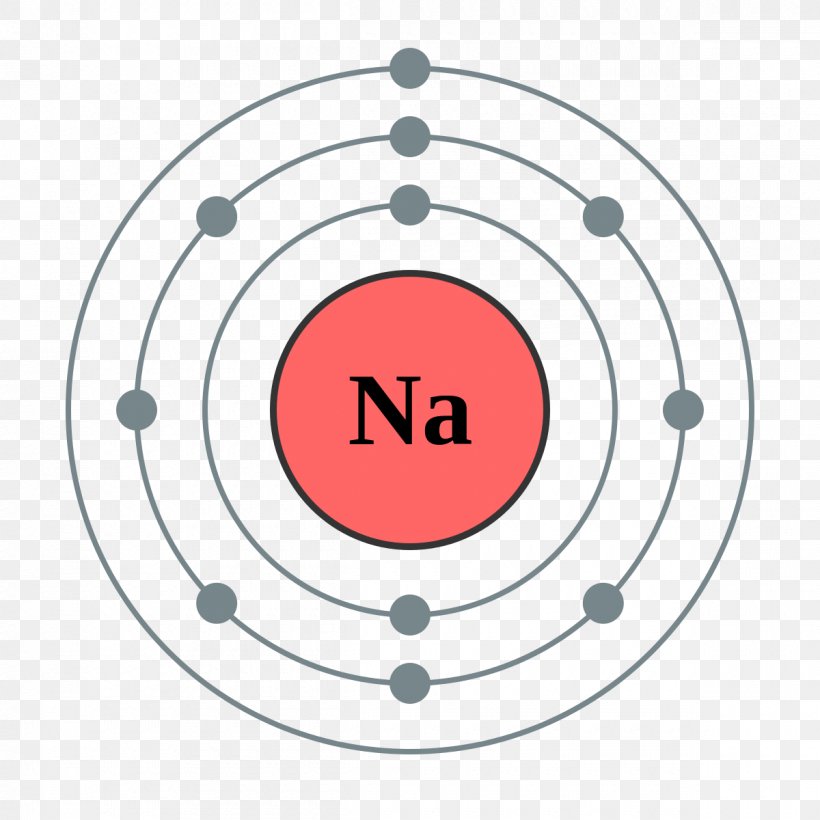
An atom of sodium has 11 electrons. The first two fill the innermost energy level. The second energy level is also full, holding eight electrons and one electron remains in the outer energy.

Atom Sodium Model Vector & Photo (Free Trial) Bigstock
nglos324 - sodium Sodium is an alkali metal in group IA of the periodic table with atomic number 11, an atomic weight of 22.99, and a density of 0.97 Mg/m 3 . Its melting point is 97.8 C, and it boils at 892 C. The electronic configuration of Sodium is (Ne) (3s 1 ). Its atomic radius is 0.190 nm and the (+1) ionic radius is 0.95 nm.
3d render of atom structure of sodium isolated over white background Stock Photo Alamy
Atomic Structure of the Sodium Atom (Na) 28,372 views 294 The Sodium atom (Na) is commonly used for examples and practice problems in chemistry. In this video we'll look at the atomic.
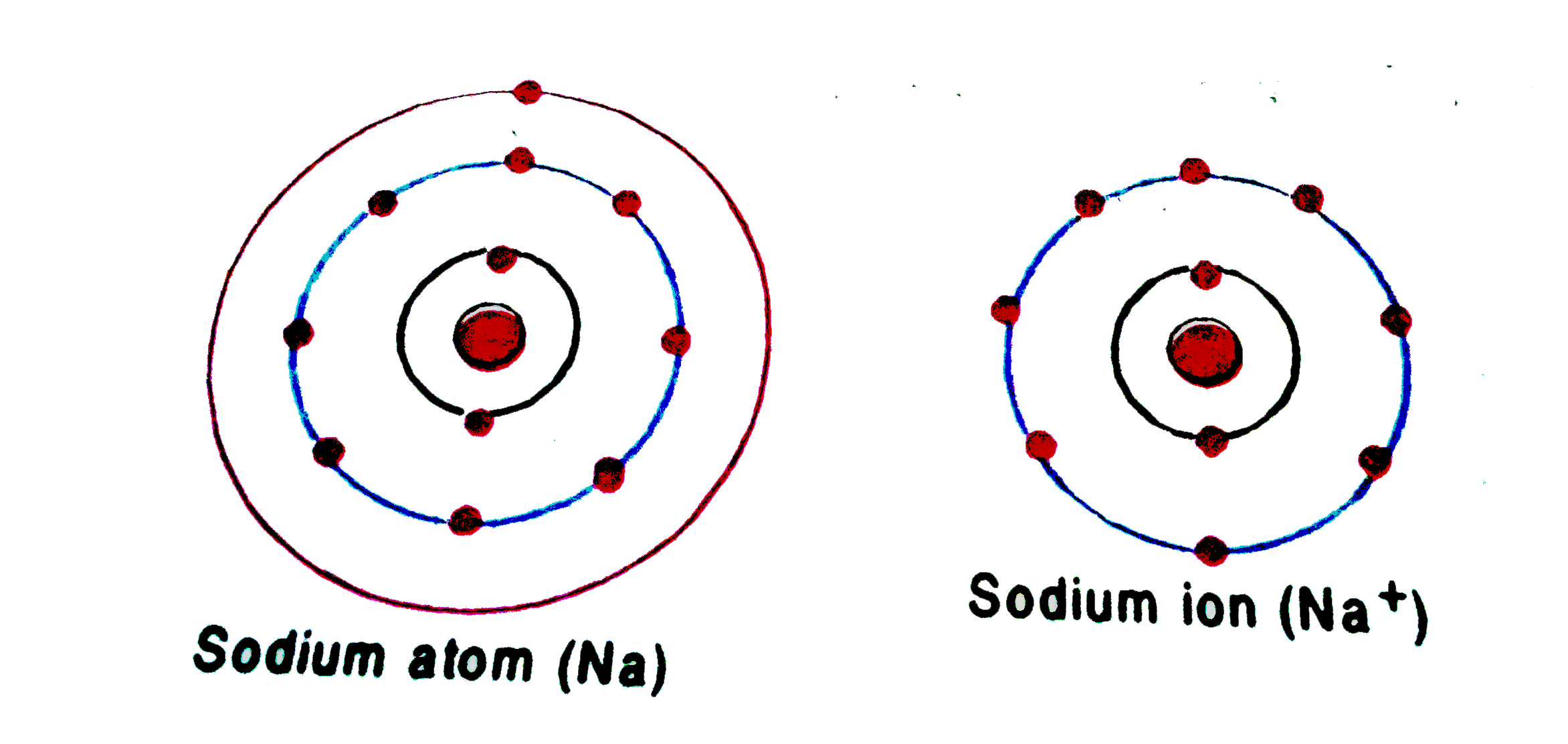
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium ion....explain why you draw
sodium (Na), chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal. Sodium is the most common alkali metal and the sixth most abundant element on Earth, comprising 2.8 percent of Earth's crust.
Electron Configuration for Sodium (Na, and Na+ ion)
Sodium has the electronic structure 1s 2 2s 2 2p 6 3s 1. When sodium atoms come together, the electron in the 3s atomic orbital of one sodium atom shares space with the corresponding electron on a neighboring atom to form a molecular orbital - in much the same sort of way that a covalent bond is formed.

Atomic structure of sodium atom Download Scientific Diagram
Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na. Sodium is a soft, silvery-white, highly reactive metal.

Sodium Atom Science Notes and Projects
Sodium (pronunciation SO-dee-em [2] ), represented by the chemical symbol or formula Na [1], is a soft, malleable element belonging to the family of alkali metals [3]. Naturally occurring Na is its most stable isotope with mass number 23 [1, 3]. Besides that, it has 16 synthetic, radioactive isotopes with known half-lives [3].