
Uranium dioxide Podcast Chemistry World
A process is outlined for producing pure UO/sub 2/ from impure oxide, e.g., uranium ore concentrate. The process comprises the steps of preparing a suspension of the impure oxide in a melt of at least one alkali and/or alkaline earth metal chIorides, chlorinattng the suspension in presence of carbon, converting UCl/sub 4/ to UO/sub 2/Cl/sub 2/ by contacting the melt with O/sub 2/, and.

Uraninite (a) uranium oxide particles in suspension preparation (TEM),... Download Scientific
In the dry process, uranium oxide concentrates are first calcined (heated strongly) to drive off some impurities, then agglomerated and crushed. At Converdyn's US conversion plant, U 3 O 8 is first made into impure UF 6 and this is then refined in a two-stage distillation process. UF 6, particularly if moist, is highly corrosive.

Figure D.5. Agitated—No pH Adjustment—5050 Uranium Oxide... Download Scientific Diagram
Specifically, the following processes are described: (1) refining/conversion of uranium in preparation of materials for natural nuclear fuel manufacturing or enrichment, (2) enrichment and (3) conversion of enriched uranium in the preparation of materials for enriched nuclear fuel manufacturing. Next chapter Keywords Nuclear fuel fuel cycle uranium

[PDF] Determination of elemental impurities and U and O isotopic compositions with a view to
Ion Exchange —This method for producing an impure uranium concentrate has been studied in considerable detail for the past ten years. Most of the plants constructed on the Colorado Plateau during the past three years are employing ion exchange. It can be used successfully for treatment of either clarified liquors or for solutions containing solids.
Raman spectrum of uranium oxide on a uranium substrate. Download Scientific Diagram
The dissolution of impure uranium oxide has been done using strong nitric acid solution [10, 11] and other different acidic media, such as nitric, sulfuric, and phosphoric acids . However, the use of alkaline solutions is also an obvious alternative to acidic UO 2 dissolution where hydroxide has been used in the presence of an oxidant such as.

PPT Lecture 6 Uranium Chemistry PowerPoint Presentation, free download ID2954973
A new x-ray study of two uranium oxides reveals an oxidation state of uranium that had not previously been observed directly. The results, reported in Physical Review Letters, should improve models for how uranium behaves in geochemical processes and how it might work as a catalyst for industrial purposes.

PPT Optical Properties of Thinfilm Uranium Oxide in the XUV PowerPoint Presentation ID6315460
Six uranium oxides were dissolved and studied; • The Gibbs free energies of all the reactions ΔrG° (T) were analyzed by the Ulich model; • Three reaction order rate models were applied on the predominant reaction reported above. Abstract

Uranium Alchetron, The Free Social Encyclopedia
Water solubility of uranium-oxide-based nuclear waste increases by 6 orders of magnitude from U(IV) to U(VI) 34, so that the oxidation during storage of the initial UO 2+x is an important safety.

Concentrations of impurities in UEu mixed oxides using ICPMS Download Table
Pitchblende is an impure uranium oxide, consisting partly of the most reduced oxide uraninite (UO 2) and partly of U 3 O 8.
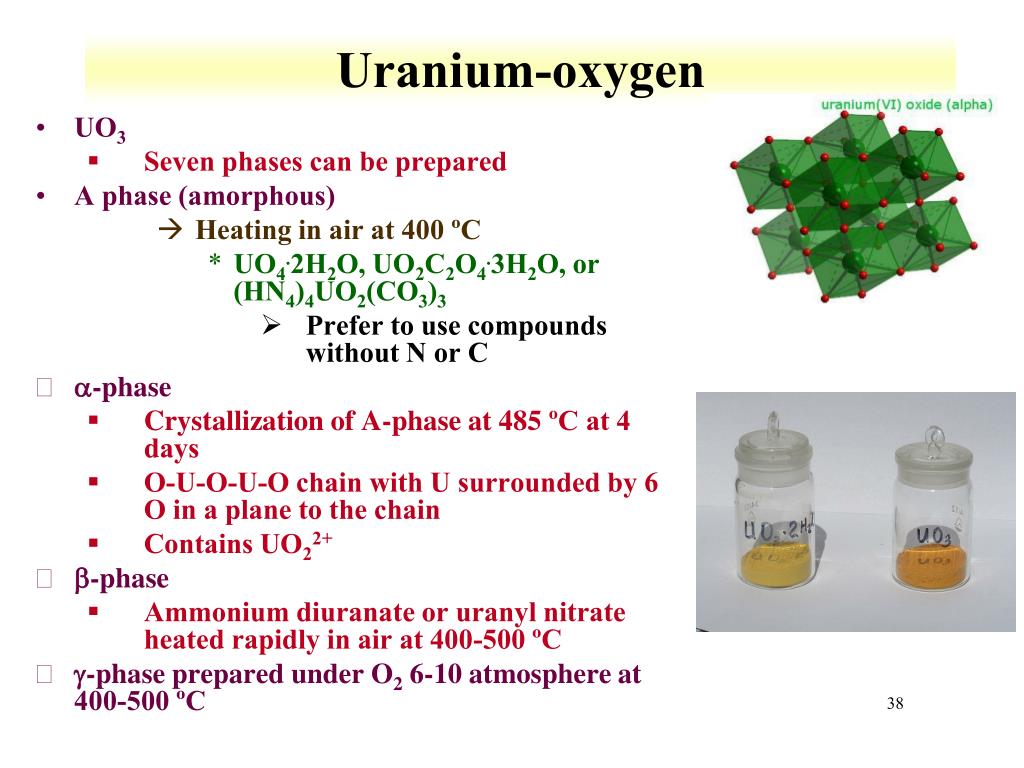
PPT RFSS Lecture 11 Uranium Chemistry and the Fuel Cycle PowerPoint Presentation ID2238985
Uranium, radioactive chemical element of the actinoid series of the periodic table, atomic number 92. It is an important nuclear fuel.. (impure U 3 O 8), uraninite (UO 2),. and +6 (as in the oxide UO 3, the hexafluoride UF 6, and the yellow uranyl ion UO 2 2+). In an aqueous solution uranium is most stable as the uranyl ion, which has a.

Energy dependence of secondary ion production from uranium oxide at... Download Scientific Diagram
Continuance of interaction of uranium oxides with liquids showed up to have more influence on oxide transformation than lower pH in gastrointestinal fluids. The structure of α-UO 3 oxide remained.

PPT Determining Optical Properties of Uranium Oxide PowerPoint Presentation ID616029
Uraninite is a uranium oxide mineral and the most important ore of uranium. It received its name from its uranium content. Uraninite is highly radioactive and should be handled and stored with care. It is not a suitable mineral for classroom use. Uraninite has an ideal chemical composition of UO 2, but the mineralogical and chemical composition.

(PDF) Determination of impurities in uranium oxide by inductively coupled plasma mass
Changes in the visual characteristics of uranium oxide surfaces and morphology following storage under different conditions of temperature and relative humidity may provide insight into the history of an unknown sample. Sub-samples of three α-U3O8 materials—one that was phase-pure and two that were phase-impure—were stored under controlled conditions for two years. Scanning electron.

Identification and Elemental Impurity Analysis of Heterogeneous Morphologies in Uranium Oxides
Uranium Hazards and Wastes. The path from raw uranium ore to a form ready for final use in piles, reactors, or the enrichment process flows through many branching steps. The first step, called milling, changed raw ore into uranium concentrate, either black oxide (uranium oxide, U3O8) or soda salt (sodium diruanate, Na2U2O7), also known as.

Impure Uranium metal obtained from pitchblende ore (8590) elementcollection
Uranium oxide is an oxide of the element uranium . The metal uranium forms several oxides: Uranium dioxide or uranium (IV) oxide (UO 2, the mineral uraninite or pitchblende) Diuranium pentoxide or uranium (V) oxide (U 2 O 5) Uranium trioxide or uranium (VI) oxide (UO 3)
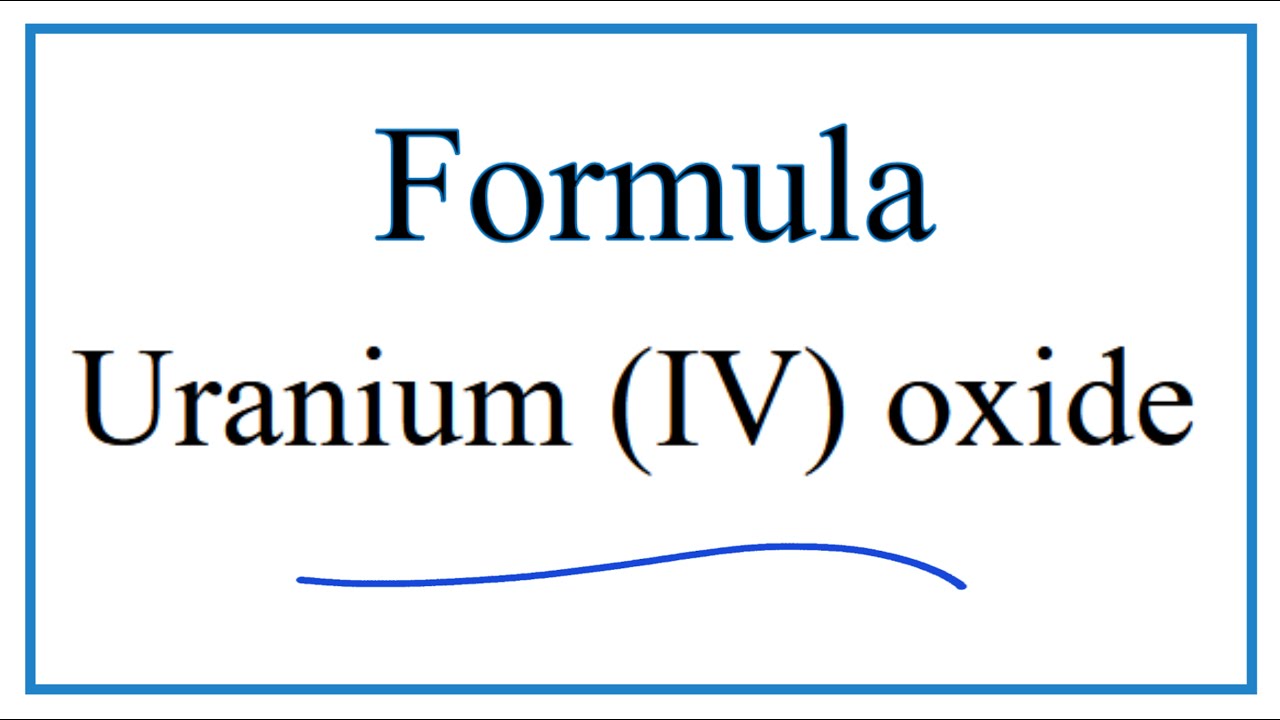
How to Write the Formula for Uranium (IV) oxide YouTube
Item: Impure Mixture of Plutonium Oxide and Uranium Oxide (PUUOXBC05) Los NATIONAL LABORATORY Alamos Los Alamos National Laboratory is operated by the University of California for the United States Department of Energy under contract W-7405-ENG-36. ii An Affirmative Action/Equal Opportunity Employer